Explain the Difference Between Alpha Beta and Gamma Radiation
Substances that give out radiation are said to be radioactive. Beta decay comes in 2 varieties.
What Is The Difference Between Alpha And Gamma Radiation
This video explains about Alpha radiation.
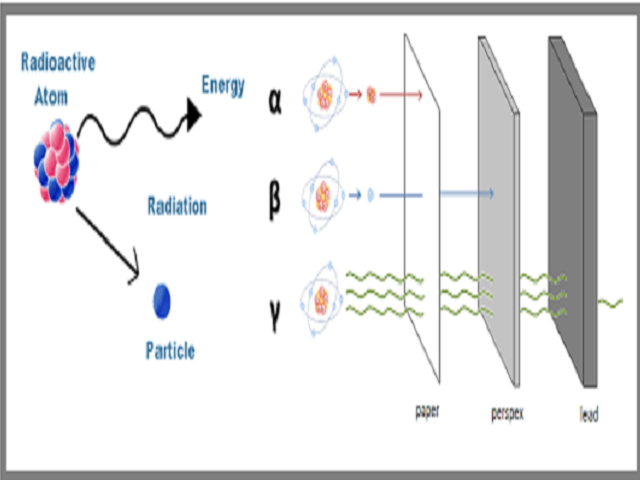
. Irradiation is about the radiation itself like alpha beta or gamma. Alpha particles are He-4 nuclei and beta is either electrons or positrons. These particles consist of two protons and two neutrons and are the heaviest type of radiation particle.
Since gamma rays penetrate more deeply through the body than alpha or beta particles all tissues and organs can be damaged by sources from outside of the body. There are three types of nuclear radiation. Nuclear radiation comes from the nucleus of an atom.
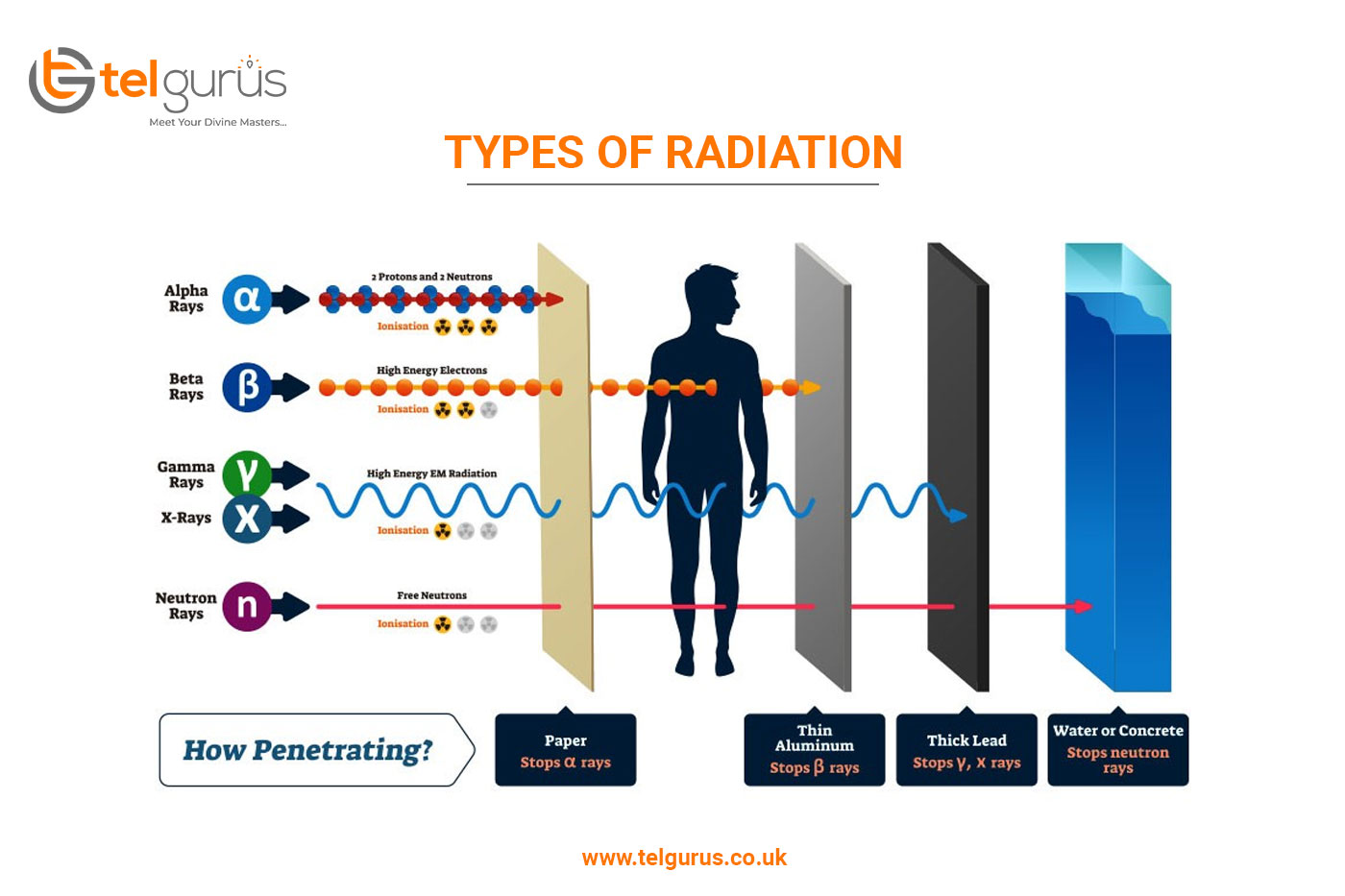
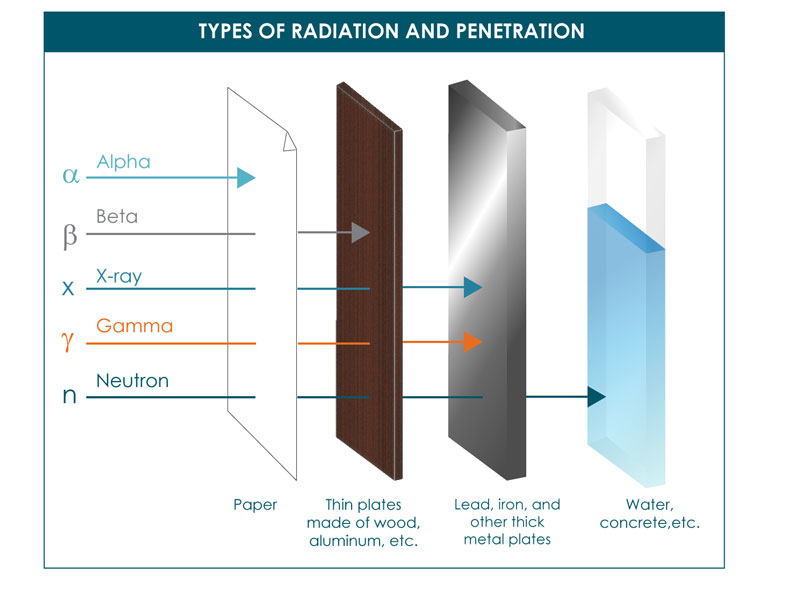
An unstable atomic nuclei loss its energy by emitting radiations such as alpha rays beta rays and gamma rays by a process called radioactive decay. Gamma rays γ are weightless packets of energy called photons. It can be stopped or absorbed by a sheet of paper.
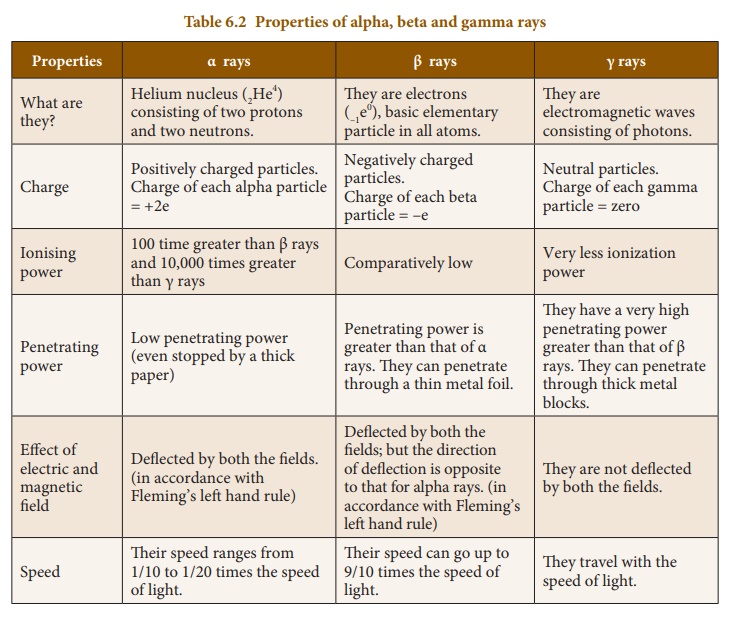
There are three major types of radioactive decay. There are four major types of radiation. Beta radiation is the producer of fast moving electrons and can penetrate further in comparison to the alpha particles.
They are more penetrating than alpha particles but. Alpha decay is the weakest type of radiation and can be stopped with a sheet of paper. Whereas a beta particle is a charged particle consisting of either unit negative or positive charge.
Beta plus a proton decays into a neutron an positron anti electron and a neutrino. Alpha - these are fast moving helium atoms. Of radioactive atoms - these are alpha beta and gamma radiation.
There are three primary types of radiation. I hope this has helped. Animation explaining the difference between contamination and irradiation.
Gamma radiation is an electromagnetic radiation and consists of high energy quanta. Beta radiation consists of free electrons or positrons at relativistic speeds which are termed beta particles. Hence the atoms eventually decay by emitting a particle that transforms when they are unstable and transforms the nucleus into a lower energy state.
Alpha and beta radiation are stream of particles consisting mass. Gamma rays are similar to visible light but have much higher energy. Gamma rays are often emitted along with alpha or beta particles during radioactive decay.
Gamma radiations are high energy radiations that are in the form of electromagnetic waves and these radiations do not give off any particle like alpha and. An alpha particle is a positively charged particle consisting of 2 protons and 2 neutrons. Alpha beta neutrons and electromagnetic waves such as gamma rays.
Unlike alpha and beta particles which have both energy and mass gamma rays are pure energy. Only sufficiently dense shielding andor distance from gamma ray emitting radioactive material can provide protection. They differ in mass energy and how deeply they penetrate people and objects.
What is the difference between Alpha Beta and Gamma Radiation. Beta minus a neutron decays into a proton an electron and an anti neutrino. A substance with such an unstable nucleus is called the radioactive substance.
Beta radiation is the producer of fast moving electrons and can penetrate further in comparison. Three types of radioation - Alpha Beta Gamma. Alpha decay involves the loss of a helium nucleus beta decay concerns protons turning into neutrons or vice versa and gamma decay involves the emission of energy without changing the original atom.
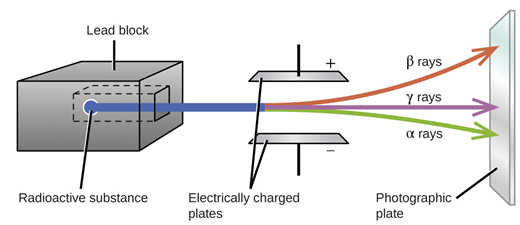
Alpha radiation is the least penetrating. Alpha decay beta decay and gamma decay. Since the gamma rays are emitted by the daughter nucleus emission of gamma rays for the emission of alpha and beta particles.
The particles produced by radioactive decay ie alpha particles beta particles and gamma rays are considerably different with distinct physical chemical and. Radiation cant travel far so is not a risk over long distances. Beta particles are between alpha particles and the third type of ionising radiation - gamma rays - in terms of how strongly ionising they are.
Alpha radiation can be described as the producer of high energy and fast moving helium particles. Alpha radiation consists of alpha particlesAn alpha particle. They carry a single negative charge.
As against a gamma particle has no charge constituent and is of neutral nature. It has a range in air of around 15cm. They are also in the middle for how penetrating they are and will pass through paper but be stopped by aluminium foil a few millimetres thick.
A question I came across asked me to explain the difference between the penetrating ability of the three different types of radiation alpha beta and gamma and explain why. The first is an alpha particle. Properties of Alpha Beta and Gamma Rays.
I know that gamma has the highest penetrating ability and alpha the smallest but I do not know the reasons behind this. Alpha and beta radiation particle radiation caused by breaking of an atom Gamma radiation electromagnetic radiation caused by movement of electrical charges. Beta particles electrons are much smaller than alpha particles.
During radioactivity particles like alpha beta gamma rays are emitted by an atom due to unstable atom trying to gain stability. Alpha particles cause lots of ionization in a short distance. They have high energy typically in the MeV range but due to their large mass they are stopped by just a few inches of air or a piece of paper.
The energy of gamma ray is equal to the difference between the energy of the excited state or higher energy state and the ground state of the nucleons. Which is the weakest type of radiation alpha or beta. Key Differences Between Alpha Beta Gamma Particles.

3 Similarities Between Alpha Beta And Gamma Rays Physics Radioactivity 13704336 Meritnation Com
Alpha Beta And Gamma Rays Properties Radioactive Displacement Law
Alpha Rays Vs Beta Rays Vs Gamma Rays What S The Difference Viva Differences

What Is The Difference Between Alpha Beta And Gamma Rays
Solved What Is The Meaning And The Differences And Similarities Of Alpha Course Hero
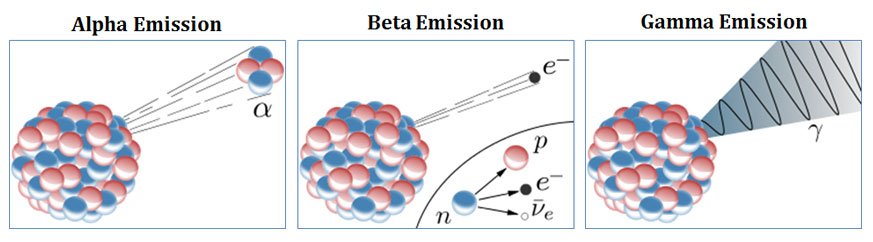
Alpha Rays Vs Beta Rays Vs Gamma Rays Compare Easy Biology Class
Do Background Radiation Consist Of Alpha Beta And Gamma Particles Quora
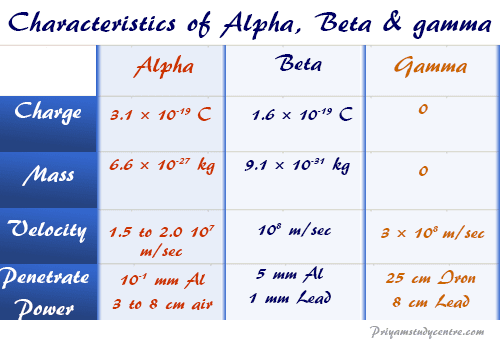
Alpha Beta Gamma Particle Properties Emission Charge Mass

Types Of Radioactivity Alpha Beta And Gamma Decay 37 Download Scientific Diagram
Difference Between Alpha Beta And Gamma Particles Definition Properties Emission Mechanism Applications
What Is The Difference Between Alpha Beta And Gamma Radiation Quora

Alpha Beta And Gamma Physics Gcse

Properties Of Alpha Beta And Gamma Rays And Differences
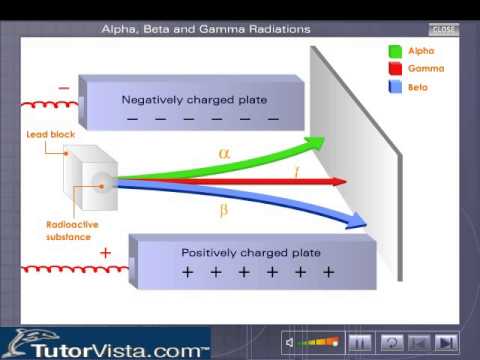
Alpha Beta And Gamma Radiations Youtube
Difference Between Alpha Beta And Gamma Radiation
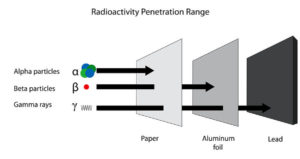
Comparison Of Alpha Particles Beta Particles And Gamma Rays

Properties Of Alpha Beta Gamma Radiation Physics Classroom Radiation Technology Wallpaper

Comments
Post a Comment